
Copyright © Luohe (Wuxi) fluid control Co., Ltd All rights reserved.
ICP2021014876

Copyright © Luohe (Wuxi) fluid control Co., Ltd All rights reserved.
ICP2021014876
The properties of steam explained here, including the ability of steam under pressure to carry, and then give up, large amounts of energy. Topics include saturated steam tables, dryness fraction and flash steam.
A better understanding of the properties of steam may be achieved by understanding the general molecular and atomic structure of matter, and applying this knowledge to ice, water and steam.
A molecule is the smallest amount of any element or compound substance still possessing all the chemical properties of that substance which can exist. Molecules themselves are made up of even smaller particles called atoms, which define the basic elements such as hydrogen and oxygen
The specific combinations of these atomic elements provide compound substances. One such compound is represented by the chemical formula 2O, having molecules made up of two atoms of hydrogen and one atom of oxygen.
The reason water is so plentiful on the earth is because hydrogen and oxygen are amongst the most abundant elements in the universe. Carbon is another element of significant abundance, and is a key component in all organic matter.
Most mineral substances can exist in the three physical states (solid, liquid and vapour) which are referred to as phases. In the case of H2O, the terms ice, water and steam are used to denote the three phases respectively.
The molecular arrangement of ice, water, and steam is still not fully understood, but it is convenient to consider the molecules as bonded together by electrical charges (referred to as the hydrogen bond). The degree of excitation of the molecules determines the physical state (or phase) of the substance.
TRIPLE POINT
All the three phases of a particular substance can only coexist in equilibrium at a certain temperature and pressure, and this is known as its triple point.
The triple point of H2O, where the three phases of ice, water and steam are in equilibrium, occurs at a temperature of 273.16 K and an absolute pressure of 0.006 112 bar. This pressure is very close to a perfect vacuum. If the pressure is reduced further at this temperature, the ice, instead of melting, sublimates directly into steam.
Ice
In ice, the molecules are locked together in an orderly lattice type structure and can only vibrate.
In the solid phase, the movement of molecules in the lattice is a vibration about a mean bonded position where the molecules are less than one molecular diameter apart.
The continued addition of heat causes the vibration to increase to such an extent that some molecules will eventually break away from their neighbours, and the solid starts to melt to a liquid state. At atmospheric pressure, melting occurs at 0 ¡ãC. Changes in pressure have very little effect on the melting temperature, and for most practical purposes, 0 ¡ãC can be taken as the melting point. However, it has been shown that the melting point of ice falls by 0.0072 ¡ãC for each additional atmosphere of pressure. For example, a pressure of 13.9 bar g would be needed to reduce the melting temperature by 0.1 ¡ãC.
Heat that breaks the lattice bonds to produce the phase change while not increasing the temperature of the ice, is referred to as enthalpy of melting or heat of fusion. This phase change phenomenon is reversible when freezing occurs with the same amount of heat being released back to the surroundings.
For most substances, the density decreases as it changes from the solid to the liquid phase.
However, H2O is an exception to this rule as its density increases upon melting, which is why ice floats on water.
Water
In the liquid phase, the molecules are free to move, but are still less than one molecular diameter apart due to mutual attraction, and collisions occur frequently. More heat increases molecular agitation and collision, raising the temperature of the liquid up to its boiling temperature.
Enthalpy of water, liquid enthalpy or sensible heat (hf) of water
This is the heat energy required to raise the temperature of water from a datum point of 0 ¡ãC to its current temperature.
At this reference state of 0 ¡ãC, the enthalpy of water has been arbitrarily set to zero. The enthalpy of all other states can then be identified, relative to this easily accessible reference state.
Sensible heat was the term once used, because the heat added to the water produced a change in temperature. However, the accepted terms these days are liquid enthalpy or enthalpy of water.
At atmospheric pressure (0 bar g), water boils at 100 ¡ãC, and 419 kJ of energy are required to heat 1 kg of water from 0 ¡ãC to its boiling temperature of 100 ¡ãC. It is from these figures that the value for the specific heat capacity of water (Cp) of 4.19 kJ/kg ¡ãC is derived for most calculations between 0 ¡ãC and 100 ¡ãC.
Steam
As the temperature increases and the water approaches its boiling condition, some molecules attain enough kinetic energy to reach velocities that allow them to momentarily escape from the liquid into the space above the surface, before falling back into the liquid.
Further heating causes greater excitation and the number of molecules with enough energy to leave the liquid increases. As the water is heated to its boiling point, bubbles of steam form within it and rise to break through the surface.
Considering the molecular arrangement of liquids and vapours, it is logical that the density of steam is much less than that of water, because the steam molecules are further apart from one another. The space immediately above the water surface thus becomes filled with less dense steam molecules.
When the number of molecules leaving the liquid surface is more than those re-entering, the water freely evaporates. At this point it has reached boiling point or its saturation temperature, as it is saturated with heat energy.
If the pressure remains constant, adding more heat does not cause the temperature to rise any further but causes the water to form saturated steam. The temperature of the boiling water and saturated steam within the same system is the same, but the heat energy per unit mass is much greater in the steam.
At atmospheric pressure the saturation temperature is 100 ¡ãC. However, if the pressure is increased, this will allow the addition of more heat and an increase in temperature without a change of phase.
Therefore, increasing the pressure ef fect ively increases both the enthalpy of water, and the saturation temperature. The relationship between the saturation temperature and the pressure is known as the steam saturation curve (see Figure 2.2.1).

Water and steam can coexist at any pressure on this curve, both being at the saturation temperature. Steam at a condition above the saturation curve is known as superheated steam:
If the steam is able to flow from the boiler at the same rate that it is produced, the addition of further heat simply increases the rate of production. If the steam is restrained from leaving the boiler, and the heat input rate is maintained, the energy flowing into the boiler will be greater than the energy flowing out. This excess energy raises the pressure, in turn allowing the saturation temperature to rise, as the temperature of saturated steam correlates to its pressure.
Enthalpy of evaporation or latent heat (hfg)
This is the amount of heat required to change the state of water at its boiling temperature, into steam. It involves no change in the temperature of the steam / water mixture, and all the energy is used to change the state from liquid (water) to vapour (saturated steam).
The old term latent heat is based on the fact that although heat was added, there was no change in temperature. However, the accepted term is now enthalpy of evaporation.
Like the phase change from ice to water, the process of evaporation is also reversible. The same amount of heat that produced the steam is released back to its surroundings during condensation, when steam meets any surface at a lower temperature.
This may be considered as the useful portion of heat in the steam for heating purposes, as it is that portion of the total heat in the steam that is extracted when the steam condenses back to water.
Enthalpy of saturated steam, or total heat of saturated steam
This is the total energy in saturated steam, and is simply the sum of the enthalpy of water and the enthalpy of evaporation.
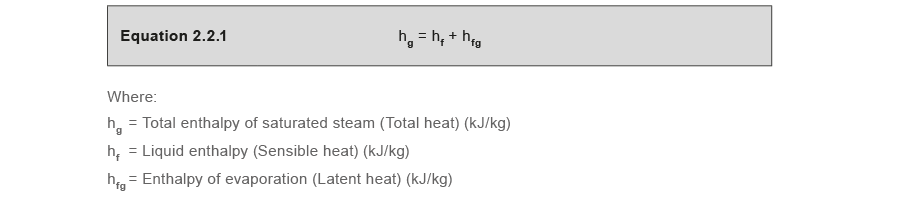
The enthalpy (and other properties) of saturated steam can easily be referenced using the tabulated results of previous experiments, known as steam tables.